Customer Profile

Optomed, a medical technology company, was founded in 2004 in Oulu, Finland. The company is one of the leading providers of handheld fundus cameras combined with screening software solutions. The company’s cameras and software that employ artificial intelligence are transforming the diagnostic process for blinding eye diseases such as rapidly increasing diabetic retinopathy. The company has an extensive portfolio of 59 international patents protecting the technology. In 2021, Optomed’s revenue reached EUR 14,8 million. Their camera products have medical approvals in all key markets, including CE (Europe) and FDA (USA), and CFDA (China). Optomed runs sales channels in over 60 countries.
Why Optomed Chose Us
From January 1, 2020, listed companies in the European Union were required to comply with the new European Single Electronic Format (ESEF) for reporting annual financial information based on a mandate by the European Securities and Markets Authority (ESMA). The ESEF mandate introduced Inline XBRL (iXBRL) as the new format in which to prepare annual financial reports, with PDFs no longer the accepted medium. iXBRL, the more advanced version of XBRL (eXtensible Business Reporting Language), facilitates digital corporate reporting, making the analysis and comparison of financial statements easy.
In 2019, when Optomed was looking for an affordable iXBRL software solution to help them comply with the ESEF mandate, IRIS CARBON® came into the picture.
IRIS CARBON® had devised a ‘Small-Company-Offer’ for companies with a market capitalization of less than Euro 100 million. Eligible companies would be offered our ESEF reporting solution and services free of charge in the first year of compliance. A great set of features, along with an astounding offer, helped Optomed decide to go with IRIS CARBON®.
Business Challenges
Optomed had the following challenges to face before complying with the ESEF mandate.
1. Dealing With A New Regulatory Mandate
The newly introduced ESEF mandate came with lots of unknowns. Optomed required an ESEF solution that was intuitive and comprehensive, as well as backed by XBRL specialists to guide their team through the contours of the new mandate.
2. No Familiarity With The New iXBRL Format
Like other EU companies, Optomed was starting out with zero knowledge of the iXBRL format. The process of converting annual reports into iXBRL by using the ESEF taxonomy to tag their disclosures, create extension elements for company-specific disclosures, anchoring the extensions, and running validation checks was an unfamiliar one for team Optomed. The company was, therefore, looking for a software provider with a background and experience in working with XBRL.
3. Meeting Filing Deadlines In A New Format
With the filing deadline approaching, Optomed needed an ESEF compliance software solution/service that could be trusted to help them meet their regulatory compliance requirements. IRIS CARBON® came with an unbeatable experience. The XBRL experts in team IRIS CARBON® have an average experience of more than 10 years and are well-versed with IFRS standards as well as the newly introduced ESEF taxonomy.
Project Objectives
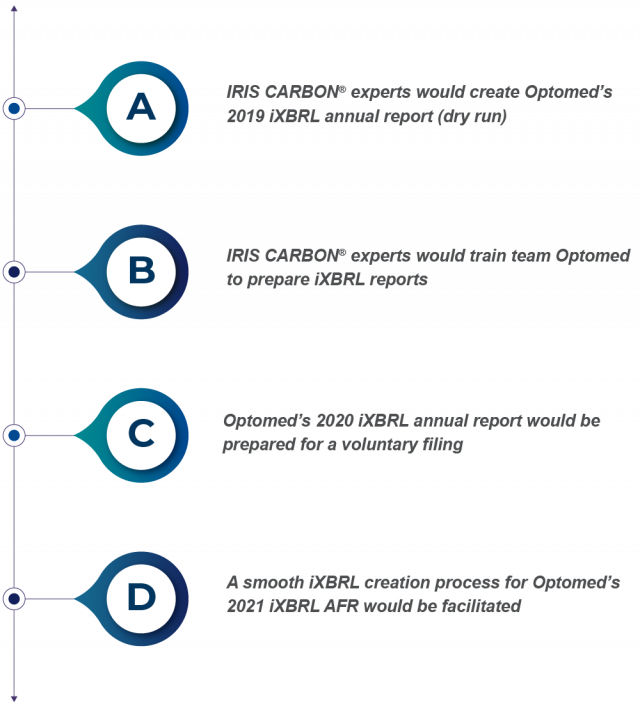
With the above-mentioned challenges in view, IRIS CARBON® and Optomed defined the following project objectives for ESEF reporting:
The IRIS CARBON® Approach
IRIS CARBON® handled Optomed’s ESEF reporting by aligning with the above-identified objectives.
1. 2019 iXBRL Creation By IRIS CARBON® (Dry Run)
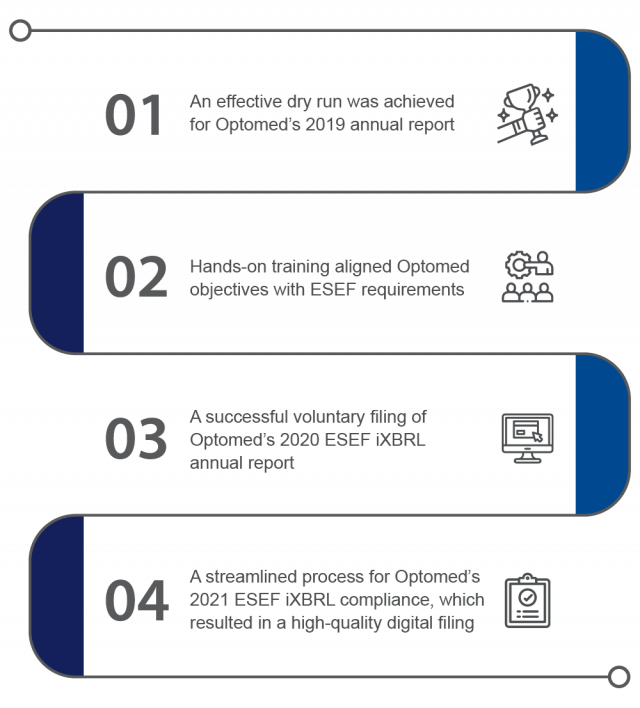
IRIS CARBON® started by doing a dry run for Optomed using their published 2019 annual report. The dry run process involved loading Optomed’s 2019 annual report onto the IRIS CARBON® platform. From the latest ESEF taxonomy (English and Finnish versions), the appropriate tags were selected and applied to Optomed’s 2019 annual report. To represent line items that did not have appropriate ESEF taxonomy tags, the experts created extensions. These extensions were then further defined by anchoring relationships. IRIS CARBON® even validated the files to make sure they were error-free.
2. Training Optomed Team In The Compliance Process
IRIS CARBON® trained team Optomed at two levels:
a) Introduction Of XBRL, iXBRL, and ESEF Process
The substance of the training IRIS CARBON® provided team Optomed included the basics of XBRL and iXBRL, the difference between the two, and the new ESEF mandate.
IRIS CARBON® walked Optomed through the ESEF taxonomy and important concepts about reviewing an annual report. This helped team Optomed efficiently review the tags in their newly tagged 2019 iXBRL annual report. IRIS CARBON® also had tips to share about validating iXBRL files, checking for errors or warning signs, and generating high-quality output files for the final submission. Team Optomed was now past basic knowledge of the ESEF mandate.
b) Hands-On Sessions Of IRIS CARBON® Platform
Once a successful dry run was done, with Optomed’s 2019 iXBRL annual report created, team IRIS CARBON® planned practical sessions around it. The sessions were designed to make team Optomed comfortable using the IRIS CARBON® platform.
They were given an overview of the IRIS CARBON® dashboard to help track the progress in reviewing the 2019 iXBRL file. The XBRL tags in the file were checked using the platform’s review module. The client team used the ‘commenting’ feature to communicate change requests (if any) in the iXBRL 2019 annual report. The hands-on training sessions came to an end with Optomed being able to review and validate their 2019 report and also generate a valid ESEF iXBRL page.
3. Creation Of The 2020 iXBRL For Voluntarily Filing
Once Optomed approved the 2019 iXBRL annual report, the ground was laid for preparing the 2020 iXBRL annual report. The company’s 2020 annual report data was uploaded to the IRIS CARBON® platform. Using the rollover function, the tags approved on the 2019 report were transferred to the 2020 report – both Finnish and English versions. Additional tags, extensions, and anchoring relationships were applied, wherever necessary.
With the 2020 iXBRL report now prepared, Optomed was asked to conduct the review, suggest changes, and approve the file. The final 2020 iXBRL annual report was then audited.
Optomed filed their 2020 iXBRL annual report voluntarily in the year 2021. Optomed also published the ESEF document on their website. To access their ESEF filings click here.
4. Facilitating A Smooth 2021 iXBRL AFR For Mandatory Filing
To get started with the 2021 iXBRL file, Optomed shared its Q3 2021 interim report for tagging in Finnish and English. Keeping the interim report as a base, IRIS CARBON® developed the 2021 iXBRL report using the same approach as in 2020.
A reviewed and approved 2021 iXBRL annual report was then filed per mandatory guidelines and published on the Optomed website. Click here to access Optomed’s reports and publications page and find the 2021 ESEF annual report.
“Keep up the good work, team IRIS CARBON®. We are fully satisfied with your assistance with our ESEF tagging, along with your unlimited support in every step. The IRIS CARBON® platform is easy to use and it functions efficiently. It makes the last-minute review of the ESEF document a convenient process. I would highly recommend IRIS CARBON® for ESEF reporting.”
Mikko Ala-Kokkila
Financial Controller
Key Results Achieved
Choosing IRIS CARBON® as the iXBRL reporting and compliance solution allowed Team Optomed to meet its goals. The IRIS CARBON® platform’s user-friendliness and effective training sessions are to be credited for the following important outcomes: